BACKGROUND
Chronic myeloproliferative neoplasms (MPN) are characterized by an high risk of thrombosis and bleeding. Historically, vitamin K inhibitors (VKAs) have been the preferred oral anticoagulant therapy (OAT) for these patients (pts). However, nowadays, VKAs are being used less frequently, and direct oral anticoagulants (DOACs) are becoming more popular. Despite this trend, there is still limited evidence in the literature regarding the efficacy and safety of these drugs in the context of MPN. Specifically, there is a lack of data directly comparing VKAs and DOACs in a real-world clinical setting.
AIMS
The study aimed to conduct a real-world analysis comparing the efficacy and safety of DOACs and VKAs in patients with MPN. Both medications were prescribed for the treatment and secondary prevention of venous thromboembolism (VTE) or for the prevention of thrombosis in pts with atrial fibrillation (AF). The secondary endpoints were to assess the influence of common demographic, clinical, and hematological variables on the incidence of thrombotic events (TE) and bleeding events (BE)
METHODS
We conducted an observational retrospective monocentric study that enrolled all consecutive pts with MPN, followed at the MPN Clinic of the IRCCS Foundation S. Gerardo dei Tintori - Monza between 1993 and 2022, who received OAT with VKAs or DOACs for VTE or AF. For each pts, we collected demographic, clinical, biological, therapeutic, and hematological data. TE were categorized based on their type (arterial or venous) and location. BE were classified as minor, major, or clinically relevant non-major bleeding, according to the ISTH classification.
Thrombosis-free survival (TFS) and bleeding-free survival (BFS) were assessed from the initiation of OAT until the occurrence of the first TE or BE, respectively, and estimated using Kaplan-Meier analysis. The other analyses were conducted using common statistical methods. Multivariable analyses included previously established risk factors for BE and TE.
RESULTS
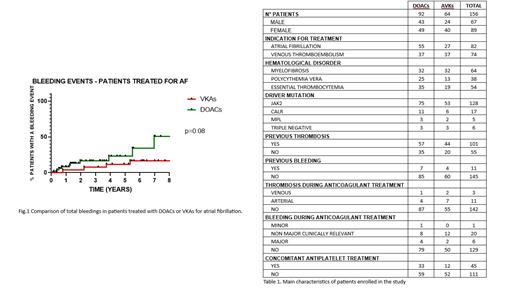
A total of 156 pts were enrolled: 89 were women (57%). The median age was 69 years (range 25-92); 61 were affected by myelofibrosis, 35 by polycythemia vera, and 48 by essential thrombocythemia.
64 pts received OAT with VKAs (41%), and 92 with DOACs (59%). For 82 pts OAT was prescribed for AF (52.6%), and for 74 for VTE (47.4%). 17 pts treated with DOACs for VTE received a reduced-dose extended treatment (45.9%). The median exposure time to OAT was 5.8 years in the VKAs group (range 0.2-20 years) and 1.77 years in the DOACs group (range 0.2-10 years).
After a median follow-up of 3,2 years, 14 TE were reported: 5 (35.7%) in the DOAC-treated group, of which 4 were arterial (80%), and 9 (64.3%) in the VKA-treated group (7 arterial - 77.8%). The incidence rate of TE was 2.11 thrombosis/pts/year in the VKAs group and 2.14 thrombosis/pts/year in the DOACs group.
There were no significant differences in terms of TFS between the two groups, whether considering the entire population or analyzing the therapeutic indications separately (respectively p=0.72, p=0.53, p=0.55).
Overall, there were 27 BE: 13 in the DOACs group (51.9%), 14 in the VKAs group (48.1%). Among these, we report 8 major BE, 4 in both groups. The incidence rate of BE was 5.6/pts/year in the DOACs group and 3.3/pts/year in the VKAs group. We did not observe significant differences in terms of BFS analyzing the whole population and the VTE group (respectively p=0.13 and p=0.75), while we found a trend for lower BFS in pts treated with DOACs for AF (p=0.08, Fig. 1). In a multivariate model, adjusted for time of exposition to OAT, age, gender and antiplatelet treatment, the risk of BE was associated solely with DOACs administration (hazard ratio 3.7, p=0.01)
We found no significant differences in the risk of TE and BE when considering the various types of DOACs used. Moreover, 45 (28.8%) patients received concomitant antiplatelet therapy, and we found no significant differences in the risk of BE or TE compared to the other group (p=0.39 and p=0.41, respectively).
CONCLUSIONS
To our knowledge, this is the largest study investigating the safety and efficacy of DOACs compared to VKAs for the treatment and prevention of TE in pts with MPN and VTE or AF. Our findings suggest that DOACs are as effective as VKAs, but they appear to exhibit a trend towards a higher overall risk of BE, especially in the AF group. More data from prospective multicentric studies are necessary to validate our results.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal